A recent article spearheaded by Fang Lin, doctoral candidate at the Center for Green Chemistry and Green Engineering, describes a novel method using electrocatalysis to break down chemical “model” compounds which are commonly found in the wood component lignin. The article is titled “Development of a Ni-promoted, selective electrochemical reductive cleavage of the C–O bond in lignin model compound benzyl phenyl ether” and was published in the prestigious journal RSC Green Chemistry and can be found here: https://doi.org/10.1039/D2GC01510B.
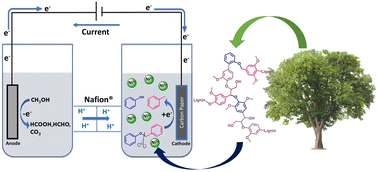
Rooted in the goal of displacing fossil-derived chemicals and fuels with those derived from biomass, this paper demonstrates the potential for breaking down large lignin molecules for their use as new chemical compounds, such as in an integrated bio-refinery. Lignin is a type of polymer found in plants and a major component in plant cell walls, specifically in lignocellulose. While it has promising potential to be used as the basis for renewably sourced chemicals, lignin polymers are complex and must be depolymerized (ie, broken down) through bond cleavage to allow for its further use.
Due to the complexity of lignin, researchers often work with so-called model compounds which contain the characteristic bonds found in lignin. Other research on lignin model compounds often includes the use of harsh conditions and/or the use of toxic reagents or precious metals. In this work Lin et al. focused specifically on one type of bond, the α-O-4 model compound benzyl phenyl ether (BPE), which could be broken via the use of a Nickel (Ni) -based catalyst in combination with electricity. Specifically, the method breaks down the model compound BPE by cleaving the benzylic C–O bond present in BPE using electrochemistry. There are several advantages to this approach: Electrochemistry can allow researchers to eschew the harsh conditions involved in traditional chemistry and align more closely with the principles of green chemistry. Electrochemistry is operated at room temperature and ambient pressure. In electrochemistry, the electric current takes the place of a stoichiometric amount of a redox agent, thus avoiding extra reagent input (e.g, H2). Nickel is an earth-abundant and relatively cheap metal, thereby lending itself as a good candidate for catalysis, and not contributing to the depletion of other, scarcer resources.
The researchers’ successful use of a Nickel catalyst to electrocatalytically cleave the C-O bond in this lignin compound in mild conditions add another technique to the toolbox on the path to realizing the integrated biorefinery, in which all parts of (renewable) biomass is valorized much like in a modern petroleum refinery. The importance to move toward producing chemicals, materials, and fuels from biomass and other renewable resources including CO2 cannot be understated, especially when considering the need to cease the use of climate change-exacerbating fossil fuels.
You can read the article online here: https://pubs.rsc.org/en/content/articlelanding/2022/gc/d2gc01510b/.
